Abstract
Introduction: MMB, an oral JAK1/JAK2/ACVR1 inhibitor, was evaluated in 3 RCTs (SIMPLIFY-1 [S1], SIMPLIFY-2 [S2], and MOMENTUM) in patients (pts) with high- and intermediate-risk myelofibrosis (MF). We conducted an integrated analysis of MMB to characterize its long-term safety, with pooled data from the 3 RCTs, representing a spectrum of MF disease from early (JAK inhibitor-naive) to late (JAK inhibitor-experienced) stages.
Methods: Adult pts with high- and intermediate-risk MF were randomized to receive MMB or ruxolitinib (RUX; S1); MMB or best available therapy, which was RUX in 89% (S2) of patients; and MMB or danazol (MOMENTUM). At the end of the 24-week randomized treatment period, pts in the MMB arms could continue treatment with open-label (OL) MMB, and pts in the control arms could cross over to receive OL MMB. Pts from all 3 RCTs continued to receive long-term extended access to MMB in the extended access protocol (XAP) study. Median follow-up time was 20 months in S1, 10 months in S2, and 7 months in MOMENTUM.
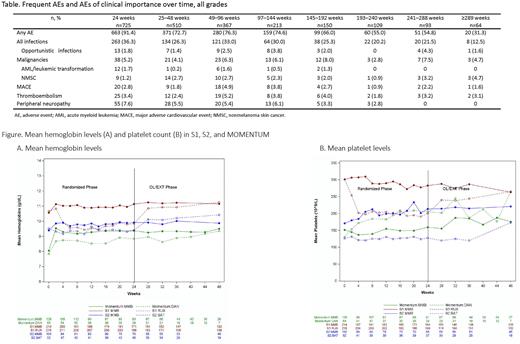
Results: As of December 3, 2021, across these 3 studies, 725 pts (1261 person-years) with MF received MMB; 12% remained on therapy for ≥5 years, with a median MMB exposure of 11.3 months, and mean (range) MMB exposure of 20.3 (0.1-90.4) months. Nonhematologic treatment-emergent adverse events (TEAEs) were mostly grade 1/2, with diarrhea (26.8% any grade, 2.6% grade ≥3) and nausea (19.4% any grade, 1.1% grade ≥3) the most frequent TEAEs, in the absence of antidiarrheal and antiemetic prophylaxis. The most frequently occurring hematologic any-grade TEAEs included thrombocytopenia (23.4%), anemia (23.0%), and neutropenia (5.7%). Infections occurred in 55.4% of patients, but serious opportunistic infections were rare (1.5%). Study drug discontinuations due to any treatment-related adverse events (AEs) were not common (15.6%). Thrombocytopenia most frequently led to study discontinuation but only occurred in 3.7% of pts. Malignancies (13.4%), including nonmelanoma skin cancer (4.8%), were infrequent. Acute myeloid leukemia (AML), or leukemic transformation, occurred in 3.0% of pts, and led to MMB discontinuation in 2.1% of pts. Peripheral neuropathy was infrequent (14.8% any grade), rarely severe (n=2 (1.2%) grade ≥3, both resolved on MMB withdrawal), mostly sensory, and rarely led to treatment discontinuation. Frequent and clinically important AEs did not increase in incidence over time (Table).
Mean baseline hemoglobin and platelet count levels were and 9.9 g/dL and 238.9 x 109/L, respectively. Hemoglobin levels were increased at first on-study assessment (week 2) on MMB and were maintained thereafter. Hemoglobin levels increased in the OL phase in pts who switched to MMB from RUX (Figure A); platelet counts improved or were maintained (Figure B).
Conclusion: We report the largest trial safety database to date for a JAK inhibitor in MF. MMB demonstrated a consistent safety profile without unexpected long-term or cumulative toxicity.
Disclosures
Verstovsek:Gilead: Research Funding; Protagonist Therapeutics: Research Funding; ItalPharma: Research Funding; PharmaEssentia: Research Funding; Incyte: Consultancy, Research Funding; Roche: Research Funding; Genentech: Research Funding; CTI BioPharma Corp.: Research Funding; Novartis: Consultancy, Research Funding; NS Pharma: Research Funding; Promedior: Research Funding; Celgene: Consultancy, Research Funding; Sierra Oncology: Consultancy, Research Funding; Constellation Pharmaceuticals: Consultancy; Blueprints Medicines Corp.: Research Funding; AstraZeneca: Research Funding; Pragmatist: Consultancy. Mesa:LaJolla Pharmaceutical: Consultancy; CTI: Research Funding; AOP: Consultancy; Promedior: Research Funding; Genotech: Research Funding; Samus: Consultancy, Research Funding; AbbVie: Research Funding; Celgene: Research Funding; Incyte: Consultancy, Research Funding; Novartis: Consultancy; Roche: Consultancy; Blueprint: Consultancy; Geron: Consultancy; Bristol Myers Squibb: Consultancy; Constellation Pharmaceuticals, Inc., a MorphoSys Company: Consultancy, Research Funding; Gilead: Research Funding; Sierra Oncology: Consultancy, Research Funding; Imago: Research Funding. Gupta:BMS Celgene: Consultancy, Honoraria, Other: Participation on a Data Safety or Advisory board; AbbVie: Consultancy, Other: Participation on a Data Safety or Advisory board; Sierra Oncology: Consultancy; Novartis: Consultancy, Honoraria; Roche: Other: Participation on a Data Safety or Advisory board; Pfizer: Consultancy, Other: Participation on a Data Safety or Advisory board; Constellation Pharmaceuticals, Inc., a MorphoSys Company: Consultancy, Honoraria. Platzbecker:BMS/Celgene: Honoraria; Takeda: Honoraria; Novartis: Honoraria; Janssen: Honoraria; Geron: Honoraria; Abbvie: Honoraria; Jazz: Honoraria; Silence Therapeutics: Honoraria. Hus:Novartis: Honoraria. Oh:Celgne/BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Kartos: Consultancy, Membership on an entity's Board of Directors or advisory committees; Sierra Oncology: Consultancy, Membership on an entity's Board of Directors or advisory committees; PharmaEssentia: Consultancy, Membership on an entity's Board of Directors or advisory committees; CTI BioPharma: Consultancy, Membership on an entity's Board of Directors or advisory committees; Disc Medicine: Consultancy, Membership on an entity's Board of Directors or advisory committees; Geron: Consultancy, Membership on an entity's Board of Directors or advisory committees; Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees; Constellation: Consultancy, Membership on an entity's Board of Directors or advisory committees; Blueprint Medicines: Consultancy, Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees. Kiladjian:BMS: Membership on an entity's Board of Directors or advisory committees; AbbVie: Membership on an entity's Board of Directors or advisory committees; Incyte: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; AOP Orphan: Membership on an entity's Board of Directors or advisory committees. Vannucchi:Morphosys: Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees; AOP Orphans Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Abbvie: Membership on an entity's Board of Directors or advisory committees; Blueprint: Membership on an entity's Board of Directors or advisory committees, Other: NA; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees; GSK: Membership on an entity's Board of Directors or advisory committees, Other: NA; Incyte: Honoraria, Membership on an entity's Board of Directors or advisory committees. Gerds:Kratos Pharmaceuticals: Research Funding; Imago BioSciences: Research Funding; Bristol Myers Squibb/Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; CTI BioPharma: Membership on an entity's Board of Directors or advisory committees, Research Funding; Incyte Corporation: Research Funding; Accurate Pharmaceuticals: Research Funding; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Sierra Oncology: Consultancy, Membership on an entity's Board of Directors or advisory committees; Morphosys/Constellation: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; PharmaEssentia: Consultancy, Membership on an entity's Board of Directors or advisory committees. Mayer:Sierra Oncology: Research Funding; Celgene: Research Funding. Sacha:Angelini: Honoraria, Membership on an entity's Board of Directors or advisory committees; Adamed: Consultancy, Honoraria; Incyte: Consultancy, Honoraria, Speakers Bureau; Pfizer: Consultancy, Honoraria, Speakers Bureau; Novartis: Consultancy, Honoraria, Speakers Bureau; Bristol Myers Squibb: Consultancy, Honoraria, Speakers Bureau; AOP Orphan: Honoraria, Speakers Bureau; BMs-Celgene: Honoraria, Speakers Bureau. Kawashima:Sierra Oncology: Current Employment. Morris:Sierra Oncology: Current Employment. Huang:Sierra Oncology: Current Employment. Harrison:Galecto: Consultancy, Membership on an entity's Board of Directors or advisory committees; Constellation Pharmaceuticals, Inc., a MorphoSys Company: Membership on an entity's Board of Directors or advisory committees, Research Funding; Sierra: Honoraria; EHA: Other: Leadership role; Gilead: Membership on an entity's Board of Directors or advisory committees; Shire: Membership on an entity's Board of Directors or advisory committees; Geron: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; AOP Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees, Other: Support for attending meetings, Research Funding; MPN voice: Other: Leadership role; Incyte: Speakers Bureau; AbbVie: Membership on an entity's Board of Directors or advisory committees; Promedior: Membership on an entity's Board of Directors or advisory committees; Roche: Consultancy, Membership on an entity's Board of Directors or advisory committees; CTI BioPharma: Membership on an entity's Board of Directors or advisory committees; Keros: Consultancy; Celgene/BMS: Membership on an entity's Board of Directors or advisory committees, Research Funding; Galacteo: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal